History
In 1667 Robert Boyle observed that following exposure to a compressed atmosphere, and subsequent decompression, an air bubble formed in the eye of a viper. The animal also appeared distressed by the experience.
In the 19th century, work began on the Brooklyn Bridge in New York. In order to build the foundations for the large stone towers of the bridge it was necessary to dig down below the surface of the riverbed. To provide a dry environment for the men working on the riverbed, underwater enclosures called caissons were used.
The air inside these underwater enclosures was pressurised to counteract the weight of the surrounding water. Following their shifts, some men would return to the surface suffering pain that made it difficult for them to stand straight. Their appearance was similar to the ‘Grecian bend’ adopted by fashionable women of the time – hence the ‘bends’.
The connection between the workers’ return to the surface and their symptoms led to the introduction of surface based recompression chambers to treat the afflicted. However, the reason for the condition was not fully recognised until 1878, when Paul Bert published his theory that the cause was the formation of nitrogen bubbles within the body. He also correctly stated that it was possible to avoid their harmful affects by ascending to the surface gradually – and that hyperbaric chambers worked, in part, because they decreased the size of bubbles.
The modern term for the ‘bends’ is Decompression sickness (DCS), which along with another diving related disorder called Cerebral Arterial Gas Embolism (CAGE) is also known as a Decompression illness (DCI).
Take a bottle of carbonated water and gently shake it. Open the bottle slowly, allowing the carbon dioxide gas dissolved in the liquid to come out of solution at a controlled rate. Few bubbles should form as a result. Now, take another bottle and repeat the above, but this time open it quickly. Many more bubbles will form. This is analogous to what happens when the body’s tissues are decompressed too quickly, although in the case of decompression sickness it is the inert gas in the breathing gas mixture that forms bubbles.
In order to appreciate the way in which inert gasses, like nitrogen and helium, can lead to decompression sickness, it is necessary to first understand a few facts.
Air consists of approximately 21% oxygen (O2) and 79% nitrogen (N2). Atmospheric pressure is caused by the weight of the air above, which in turn is the effect gravity has on the various gasses in it. This pressure decreases with altitude. The more molecules there are in a given volume of a gas, the higher the pressure.
Under the surface of a body of water, the weight of the water molecules causes hydrostatic pressure, which increases proportionally with the amount of water above (depth).
There are two ways to enable divers to breathe underwater. The first is by enclosing the diver in a protective air-filled capsule, such as submarines. The other method is to provide the diver with breathing air at the same pressure as the surrounding water.
Dalton’s law states that every gas in a mixture of gases exerts its own pressure independently of the others. The pressure of a single gas in a mixture is known as the ‘partial pressure’.
Formula
Pt = P1 + P2 + P3 …
(Where Pt is total pressure, P1 is the partial pressure of gas one, P2 of gas two, etc.)
For example, the simplified composition of air is 21% oxygen and 79% nitrogen. If the total air pressure equals 1 ATA, then the partial pressure of the two gases will be 0.21 ATA and 0.79 ATA respectively – i.e. 21% of 1 ATA equals 0.21 ATA.
At a depth of 50 msw, the pressure equals 6 ATA. Therefore, the partial pressure of nitrogen (pN2) will be 79% of 6 ATA, which is 4.74 ATA – i.e. there are six times as many N2 molecules in a SCUBA diver’s air supply at a depth of 50 msw than at the surface.
This explains how a diver is exposed to an increase in inert gas simply by breathing at depth. Henry’s law goes on to explain how the gas spreads through the body’s tissues.
When a liquid is exposed to a gas, some of the gas molecules will dissolve into it. The number of molecules that dissolve into the liquid depends on factors such as the mass of the liquid, the partial pressure of the gas, its solubility and the surface area of contact.
Oxygen is normally carried by haemoglobin and only dissolves into the liquid plasma in small amounts. If the partial pressure of oxygen is increased, as with hyperbaric therapy or SCUBA diving, then more oxygen molecules will dissolve. Unfortunately, this is also the case for other gases in a diver’s breathing gas supply – including nitrogen. Because nitrogen is not utilised by the body, it will accumulate in the tissues until they can absorb no more molecules at that pressure, i.e. until they become ‘saturated’.
The body consists of various types of tissue. The rate at which an inert gas is absorbed (loaded) by each tissue during hyperbaric exposure, and subsequently released (off-loaded or off-gassed) during decompression, depends on several factors. These include the blood perfusion in the tissue and the solubility of the gas in each particular tissue type. A simplified description of tissues is that they can be fast or slow at absorbing and releasing inert gas. The table below gives examples of the ‘speed’ at which this process can occur for several tissue types exposed to both nitrogen and helium – the two most commonly used inert gasses in diving.
Tissue |
Half-time, Nitrogen (mins) |
Half-time, Helium (mins) |
||
|
Spinal Cord |
12.5 |
12.5 |
|
|
|
Skin, Muscle |
37 – 79 |
14 – 30 |
|
|
|
Inner Ear |
146 – 238 |
55 – 90 |
|
|
|
Joints, Bones |
304 – 635 |
115 – 240 |
|
|
|
Edmonds, Lowry and Pennefather (1991) |
|
|||
As pressure is reduced, the inert gas in the tissues is carried away only slowly by the blood to the lungs where it can leave the body.
Off-gassing can be carried out more quickly by breathing an oxygen enriched atmosphere. However, oxygen becomes toxic at high partial pressures. Long term effects include pulmonary damage, but of greater concern to divers is the effect oxygen has on the central nervous system. At a pO2 greater than about 2ATA there is a risk of seizures. This equates to a depth of just 10msw, and the risk increases with depth. A pO2 limit of 1.6ATA is generally recommended for in-water diving, although much higher pO2 levels are routinely used for supervised treatment sessions in hyperbaric chambers, where there is no risk of drowning.
Boyle’s law states that if a fixed mass of a gas such as oxygen or nitrogen is compressed then the volume of that gas will decrease.
Formulae
P x V = k OR P1 x V1 = P2 x V2
(Where P1, P2 are pressure, V1, V2 are volume and k is a constant.)
For example, the volume in a diver’s lungs equals 4 litres of air at the surface. What would the volume of air be at 10 msw if the diver held his breath? Firstly, the pressure at the surface equals 1 ATA. At 10 msw it is 2 ATA.
P1 x V1 = P2 x V2
V2 = P1 x V1 / P2
V2 = 1 ATA x 4 / 2
V2 = 2 litres.
The formula can be used to show that the gas volume will continue to fall as pressure increases. Conversely, during decompression the volume of any bubbles (including microscopic ones) will increase. Note that the change in volume is greatest nearer the surface.
This can be demonstrated with a large syringe filled with a carbonated drink without obvious bubbles. Cap the syringe and pull the plunger to simulate a drop in pressure – the gas in the liquid will come out of solution to form bubbles. Notice that when the plunger is released, despite a return to the original pressure, the bubbles in the liquid remain. In fact, even when pressure is increased by pushing the plunger, the bubbles do not all immediately dissolve back into the liquid.
Rather than spontaneously forming in a liquid, bubbles are ‘seeded’ by areas of slight imperfections on a surface. This can be seen when looking at a glass of carbonated drink – bubbles tend to appear on the inside surface of the glass at the same points. The internal structure of the human body is more irregular than the seemingly smooth surface of glass, and the potential for bubble formation is greater at areas where there is damaged tissue.
Predisposing Factors
The risk of developing decompression sickness depends on many factors, most of which are not yet clearly understood. Some of these are discussed below.
Flying After Diving
Because there are fewer molecules of gas at higher altitude, there is less pressure and therefore a lower partial pressure of each gas in the air – which means there is less oxygen available. This hypobaric effect is the opposite of hyperbaric pressure.
This concept is familiar to most people since aircraft flying at high altitudes must have pressurised cabins to support life. All commercial passengers are reminded that in the event of sudden cabin decompression oxygen masks will drop down from above. In normal operation the cabin ‘altitude’ can be as high as 7,500 – 8,000 feet.
Although reducing the available amount of oxygen to the body can lead to hypoxic conditions, most healthy people will not experience symptoms during a normal commercial flight. The main risk to divers from flying is not the reduction in oxygen partial pressure, but the reduction in atmospheric pressure itself.
The degree of change in a hypobaric atmosphere is less pronounced than in a hyperbaric atmosphere, but pre-existing nitrogen bubbles in the body will grow larger and may exacerbate or even cause DCS. Larger gas spaces, such as a pneumothorax, will also grow.
The length of time required between diving and flying depends on many factors, e.g. depth, duration and number of dives, therefore it is impossible to give a definitive pre-flying period. However, it is recommended that following SCUBA diving an individual should wait at least 12 to 24 hours before flying (Divers Alert Network).
Dehydration
This is one of the main factors in increasing the severity of DCS. In order to off-load the increased burden of inert gas from the body, a good volume of fluid in the circulatory system is needed. If a casualty is dehydrated, gas transport will be inhibited by the reduction in plasma while bubble formation and growth will be encouraged by the relative increase in density of solid matter in the blood.
As bubbles grow and combine to form larger ones, they affect the surrounding tissue making it more permeable to liquid. This results in a loss of fluid from the circulation and ever increasing dehydration.
Dehydration should be avoided by drinking plenty of water following a dive. Drinking copiously before a dive may allow more inert gas to be absorbed which will increase the risk of DCS if the extra fluid is then lost prior to surfacing.
Alcohol
The effects of alcohol on co-ordination, consciousness and mental reasoning are well documented and have obvious implications in the ability of a diver to carry out the tasks necessary for a safe dive. This reduction in ability also applies to a ‘hangover’.
Alcohol will increase the chances of developing nitrogen narcosis due to the depressant effect on the central nervous system. As with any CNS condition, intoxication following a dive will potentially mask some of the symptoms.
Alcohol is also a powerful diuretic that will increase urine output and promote dehydration.
Repetitive diving
Following each dive the body retains some of the inert gas dissolved in the tissues. The correct use of dive tables usually allows enough of the gas to be excreted via the lungs to avoid the formation of bubbles large enough to cause physiological problems.
Repeated dives will increase the risk of DCS if adequate time is not allowed to offload the dissolved gas. For the same reason, the depth and duration of the dive will influence the amount of absorbed gas.
Temperature
SCUBA diving in warm water will generally result in higher nitrogen loading than in cold water. This is due mostly to the effects of vasodilatation. Becoming cold following a dive may increase urine output and lead to dehydration. In any suspected case of DCS, the casualty must be kept warm and dry.
Obesity
An increase in adipose tissue leads to an increase in nitrogen loading in the body, since nitrogen is five times more soluble in fat tissue than muscle. Because fat can absorb more gas than muscle, it will take longer for the body to excrete it. It is generally assumed that there is a related increase in risk of decompression sickness.
Obesity also increases the body’s oxygen requirements, leading to a likely reduction in physical ability. The long-term implications of obesity are well known. Conditions related to obesity such as diabetes and coronary artery disease are themselves believed to be DCS risk factors.
Exercise
Fit, healthy individuals are less likely to suffer from most medical conditions than unfit ones but there are possible risks involved with strenuous physical exercise.
It has been suggested that strenuous exercise causes the formation in the body of tiny gas bubbles called ‘micronuclei’ (Dervay et al, 2002). It takes several hours for these bubbles to disperse.
Age & Gender
There are conflicting studies showing the existence, or lack, of a relationship between a diver’s age / gender and the incidence of decompression sickness. A study by the Armstrong Laboratory concluded that there is a three-fold increase in high altitude DCS in men over 42 years old compared to those aged 18-21. The reason for any increased risk in dive related DCS might be due to the distribution and amount of fat in the diver’s body.
Patent Foramen Ovale (PFO)
One of the most significant predisposing risk factors in DCS is a physical abnormality of the heart called Patent Foramen Ovale (PFO) – a small opening between the upper two chambers of the heart (atria).
Every unborn baby has a Foramen Ovale that allows blood already oxygenated by the mother’s lungs to by-pass the baby’s respiratory system. The opening usually seals up following birth.
If it remains open (patent) then it can cause a ‘short circuit’ of blood flow allowing bubbles to pass directly from the venous circulation to the brain through the heart, bypassing the filtering effect of the respiratory system.
PFO is present in about 25% of the general population and does not normally cause health problems. However, it affects up to 75% of those with ‘unexplained’ DCS (Kerut et al, 2001).
Although PFO increases the risk of decompression sickness, individuals with PFO do not necessarily develop symptoms. Also, those who have suffered an ‘unexplained’ case of DCS do not always have a PFO.
Injury
Because bubbles form more easily around physical imperfections, tissue damage (e.g. a ‘bad knee’) can lead to greater risk of bubble formation and DCS. The risk may also be increased due to localised changes in blood perfusion.
Previous DCS
Divers who have had previous occurrences of DCS are far more susceptible to the condition than those who have never had it. This is related to tissue damage caused by previous bubble formation, especially in the Central Nervous System, and pre-existing susceptibilty to the condition.
Some divers, when questioned following an incidence of DCS, reveal that they have possibly suffered ‘sub-clinical’ bends in the past – i.e. the symptoms at the time were not obviously manifested.
Smoking
Cigarette smoke contains carbon monoxide (CO), which is a poison. This reduces the ability of the red blood cells to carry oxygen. Cigarette smoke also contains nicotine, which acts as a vasoconstrictor and may theoretically increase the risk of DCS due to altered blood perfusion.
Long term problems include chronic obstructive pulmonary disease (COPD) which leads to carbon dioxide (CO2) retention. This has been implicated as a factor in CNS oxygen toxicity. Smokers are also generally less healthy and are more prone to develop circulatory problems.
Signs and Symptoms of DCS
Diagnosis is based mainly on the patient’s history. For example, an 80 year old lady in A&E complaining of a painful shoulder, shopping bags by her trolley, is unlikely to have DCS – whereas a 20 year old with the same presenting symptoms and a SCUBA tank next to them is a likely candidate for recompression treatment.
A useful description of DCS can be achieved by noting the area(s) of the body affected.
General signs (constitutional).
- Nausea, weakness or fatigue.
Skin (cutaneous).
- Itching (pruritis).
- Generalised rash.
- Lumps.
- Cutis marmorata marbling (serious sign).
- Crackling feeling (subcutaneous emphysema) – not usually around collarbone.
Musculoskeletal (muscles and joints).
- Joint or muscle discomfort and / or pain ("bends").
- Limitation of limb movement.
- Crunching sound in joint.
Gastrointestinal (stomach and bowel).
- Nausea, vomiting.
- Abdominal cramps, diarrhoea.
Cardiorespiratory (heart and lungs).
- Coughing.
- Chest pain made worse on inspiration.
- Tachypnoea (increase in breathing rate).
Neurological (cerebrum, cerebellum, spinal cord, inner ear and peripheral nerves).
- Headache.
- Confusion.
- Memory loss.
- Tremors.
- Visual disturbance (scotoma).
- Involuntary eye movement (nystagmus).
- Lack of co-ordination (AtAxia).
- Numbness or altered sensation.
- Pins and needles (paresthesia).
- Urinary retention / incontinence.
- Ringing sound in ears (tinnitus).
- Hearing loss.
- Dizziness, loss of balance (vertigo).
- Partial or full paralysis of lower limbs (paraparesis / paraplegia).
- Unconsciousness.
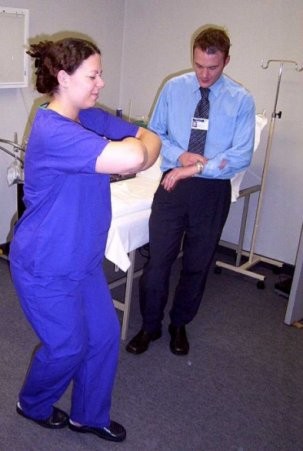 The “Rhomberg test” is commonly used to assess a patient with suspected DCS. The patient is asked to stand heel to toe, with legs slightly bent at the knees. They then cross the arms with them lifted off the chest and close their eyes. Those with neurological DCS are unlikely to be able to maintain their balance for more than a second or two.
The “Rhomberg test” is commonly used to assess a patient with suspected DCS. The patient is asked to stand heel to toe, with legs slightly bent at the knees. They then cross the arms with them lifted off the chest and close their eyes. Those with neurological DCS are unlikely to be able to maintain their balance for more than a second or two.
It must be emphasised that even in the absence of obvious symptoms, the possibility of DCS should be considered if the diver’s circumstances suggest such a risk. Those with any symptoms of DCS, however mild, are at risk of further episodes.
Classification of DCS
Decompression sickness can be classified as either Type 1 or Type 2. The different classification reflects the effect, and therefore the severity, of the condition.
Type 1 DCS can occur when bubbles affect the tissues around skeletal joints. The areas most often affected are the knees, elbows and shoulders.
Decompression sickness might also present as a skin (cutaneous) disorder. Nitrogen bubbles can cause mottling, lumps or a rash. "Skin bends", as they are colloquially termed, are more common during hyperbaric chamber ‘dives’ and when diving using a dry suit. Although not usually in themselves serious, skin symptoms may indicate the presence of problems elsewhere. If left untreated, Type 1 DCS may progress to Type 2.
Type 2 decompression sickness reflects involvement of the Central Nervous System (CNS) and / or the cardio-respiratory system. More than half of those diagnosed with DCS will be classified as Type 2. Cerebral symptoms arise from interruption of the blood supply to the main part of the brain, and include confusion, reduced mental function and unconsciousness. Involvement of the cerebellum may lead to tremors, loss of balance ("staggers") and a lack of co-ordination (ataxia). Balance may also be affected by damage to the vestibular part of the inner ear.
Spinal DCS may present as back pain, paresthesia (pins and needles), paralysis and loss of urinary sphincter control – resulting in either incontinence or retention.
As discussed already, the formation of small inert gas bubbles does not necessarily lead to the development of DCS. Likewise, when bubbles become trapped in the tiny blood vessels around the lungs’ alveoli (air sacs), problems do not always arise. In fact, it is thought that their accumulation in this area may increase the rate that the gas is excreted from the body (Edmonds et al, 1993). However, if too many bubbles collect, breathing will become adversely affected ("chokes"). Symptoms include breathlessness, tachypnoea, chest pain and coughing. Although symptoms may resolve, this should be regarded as a life-threatening condition as it may progress to fatal respiratory collapse.
Cerebral Arterial Gas Embolism (CAGE)
This is caused by rupture of the fragile lining of the lung’s alveoli allowing large quantities of air to enter the blood vessels leading to the small arteries in the brain via the heart.
Cerebral Arterial Gas Embolism is the result of pulmonary barotrauma usually caused when a diver runs out of air. This will often result in a panic reaction, with breath-holding and rapid ascent to the surface leading to over expansion of the lungs as the volume of air in them increases with decreasing ambient water pressure (Boyle’s Law).
Signs and Symptoms of CAGE
Cerebral Arterial Gas Embolism is the result of pulmonary barotrauma, which may present with the following symptoms.
- Subcutaneous emphysema, particularly around collarbone area.
- Chest pain.
- Shortness of breath (dyspnoea).
- Coughing, possibly with blood (haemoptysis).
- Increase in heart rate (tachycardia).
- Decrease in blood pressure (hypotension).
The resulting cerebral damage may result in the following symptoms.
- Severe headache.
- Paralysis.
- Numbness.
- Unconsciousness.
Symptoms similar to those of neurological DCS may also be present. Any confusion between the diagnosis of CAGE or neurological DCS should not influence the immediate care of the affected diver, as both are medical emergencies treated initially in the same way.
Treatment of DCS (CAGE and DCI)
Initial treatment for all suspected cases of decompression illness, whether thought to be DCS or CAGE, should be the same. Firstly, the accepted practice of "Safety, ABC" should be used.
- Safety first – do not place yourself at risk of becoming another casualty, it is more difficult to treat two divers than just one.
- If unconscious, place the casualty in the recovery position and ensure airway is open.
- Administer high concentration oxygen if available.
- Perform CPR if required.
- Seek medical assistance immediately- see the contact section for details.
In addition, rescuers should ensure the following.
- Lay the casualty down and keep them horizontal – this may help prevent bubble migration to the brain.
- Encourage the diver to remain calm and still.
- Protect against hypothermia – replace wet clothes with dry. Do not expose to excessive heat.
- Encourage fluid intake (aim for 1 litre in the first hour) – if the casualty has a reduced conscious level or has difficulty in swallowing then avoid giving oral fluids. If available, intravenous fluid therapy is preferred.
- Monitor for deterioration and record observations.
- Notify the appropriate emergency service of any deterioration.
- Do not give opiates as this may reduce respiratory rate and prolong nitrogen off-loading.
- Never administer Entonox ("gas and air"), as this will worsen the condition due to its high nitrogen content.
Recompression Therapy
The definitive treatment for any form of decompression illness is recompression in a hyperbaric chamber.
The main purpose of this is to reduce the size of any existing bubbles. In addition, 100% oxygen is given in order to encourage the excretion of nitrogen from the body.
Symptoms may persist due to existing tissue damage and the accumulation of blood cells, etc, around the points where bubbles were situated.
At the Hyperbaric Medicine Unit in Aberdeen the most common therapy for bends is based on a modified U.S. Navy Table 6 profile. In this treatment, the patient is compressed to nearly three times normal atmospheric pressure, which equates to a depth of 18msw.
Depending on the patient’s condition during the treatment, the table may be extended, or changed to a Heliox (HeO2) saturation table lasting several days.
References
Heymans O, Wuilmart M, Gielen JL
[Scuba diving: risks for whom and why?]
Rev Med Liege (Belgium), Apr 2001, 56(4) p248-52
Gustavsson LL, Hultcrantz E
[Medical aspects of diving–a sport for both women and men]
Lakartidningen (Sweden), Feb 17 1999, 96(7) p749-53
Carraway MS, Piantadosi CA
Oxygen toxicity.
Respir Care Clin N Am (United States), Jun 1999, 5(2) p265-95
Turle-Lorenzo N, Zouani B, Risso J
Narcotic effects produced by nitrous oxide and hyperbaric nitrogen narcosis in rats performing a fixed-ratio test.
Physiol Behav (United States), Sep 1999, 67(3) p321-5
Edmonds C, Lowry C, Pennefather J
Diving and Subaquatic Medicine.
3rd Edn, Butterworth Heineman, 1991.
Schwerzmann M, Seiler C
Recreational scuba diving, patent foramen ovale and their associated risks.
Swiss Med Wkly (Switzerland), Jun 30 2001, 131(25-26) p365-74
Wilmshurst P, Bryson P
Relationship between the clinical features of neurological decompression illness and its causes.
Clin Sci (Lond) (England), Jul 2000, 99(1) p65-75
